Microtubules are conveyer belts inside the cells. They move vesicles, granules, organelles like mitochondria, and chromosomes via special attachment proteins. They also serve a cytoskeletal role. Structurally, they are linear polymers of tubulin which is a globular protein. These linear polymers are called protofilaments. The figure to the left shows a three dimensional view of a microtubule. The tubulin molecules are the bead like structures. They form heterodimers of alpha and beta tubulin. A protofilament is a linear row of tubulin dimers.
Microtubules may work alone, or join with other proteins to form more complex structures called cilia, flagella or centrioles . In this unit we will cover all of these structures.
Note: many of the photos are from the text, or from Histology texts by Bloom and Fawcett used by our students. They are for illustration at this site only and for individual student use.

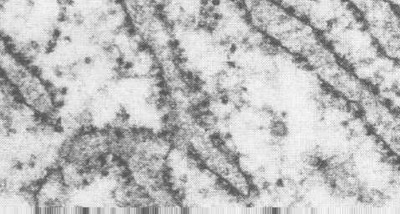
Microtubules can be seen in a bundle in the above negatively stained preparation. Recall that negative staining starts by immobilizing the preparation on plastic on an electron microscopic grid. Then heavy metal stain is deposited around the structures, delineating their structure. This preparation may allow you to see the tubulin molecules in the protofilaments. (Taken from Bloom and Fawcett; Textbook of Histology)

This transmission electron micrograph to the right shows the microtubules in longitudinal ultrathin section. Note, the tubulin molecules cannot be visualized in this preparation.
Early electron microscopists found that in order to preserve microtubules, they had to fix the cells in glutaraldehyde at room temperature. Why do you think the temperature conditions were important? What might happen if they fixed the cells for 30 min in the cold?


The extensive distribution of microtubules can really be appreciated in the light microscope after immunolabeling for tubulin with fluorescein-labeled antibodies. This micrograph shows cells in culture labeled for tubulin. The labeling is so fine, the small microtubules can be delineated.
Microtubule Formation


The first stage of formation is called "nucleation". The process requires tubulin, Mg++ and GTP and also proceeds at 37 C. This stage is relatively slow until the microtubule is initially formed. Then the second phase, called "elongation" proceeds much more rapidly.
During "nucleation", an alpha and a beta tubulin molecule join to form a heterodimer. Then these attach to other dimers to form oligomers which elongate to form protofilaments. Each dimer carries two GTP molecules. However the GTP that appears to function binds to the beta tubulin molecules. When a tubulin molecule adds to the microtubule, the GTP is hydrolyzed to GDP. Eventually the oligomers will join to form the ringed microtubule. The hydrolysis of GTP of course is facilitated at a temperature of 37 C and stopped at temperatures of 4 C.
The figure above shows that, as the oligomers assemble, they form a series of rings, 25 nm in diameter. In cross section, each ring consists of 13 beads. The rows of beads in longitudinal section are called protofilaments. Look at your text figures for the series on assembly of MT from protofilaments.
In the cell itself, microtubules are formed in an area near the nucleus called the "aster". This is also called the Microtubule Organizing Center (MTOC). Microtubules are polar with a plus end (fast growing) and a minus end (slow growing). Usually the minus end is the anchor point in the MTOC. In this figure, the plus end is shown to the left by the numerous tubulin dimers. This is the end that carries the GTP molecules which may be hydrolyzed to GDP.
Hydrolysis is not necessary, however . Tests have shown that microtubules will form normally with nonhydrolyzable GTP analog molecules attached. However, they will not be able to depolymerize (see below). Thus, the normal role of GTP hydrolysis may be to promote the constant growth of microtubules as they are needed by a cell.
Dynamic instability
Microtubules may vary in their rate of assembly and disassembly. Tubulin half life is nearly a full day, however, the half life of a given microtubule may be only 10 minutes. Thus, they are in a continued state of flux. This is believed to respond to the needs of the cell and is called "dynamic instability". Furthermore, there are regulatory processes that appear to control this in a cell. Microtubule growth would be promoted in a dividing or moving cell. However, microtubule growth would be more controlled in a stable, polarized cell.
As described in your text, the cell can provide a GTP cap on the growing end of a microtubule to regulate further growth. This happens when the tubulin molecules are added faster than the GTP can be hydrolyzed. Thus, the microtubule becomes stable and does not depolymerize. It may also be encouraged to continue growing. Once the GTP is hydrolyzed, it begins to shrink, however. Another way of capping a microtubule is to put a structure at its end, such as a cell membrane.
How Microtubule Associated Proteins (MAPs) function.

Microtubule associated proteins (MAPs) are tissue and cell type specific. Assembly MAP's are high molecular weight proteins (200-300 K) or the tau (20-60 k) proteins. One domain binds to tubulin polymers or unpolymerized tubulin. This speeds up polymerization, facilitates assembly and stabilizes the microtubules. The other end projects out and will bind to vesicles or granules, IF or other MT.
Assembly MAP’s are high molecular weight proteins (200-300 K) or the tau (20-60 k) proteins. One domain binds to tubulin polymers or unpolymerized tubulin. This speeds up polymerization, facilitates assembly and stabilizes the microtubules. The other end projects out and will bind to vesicles or granules, IF or other MT.
Type I (MAP IA, IB)
- Binding domain: Contains “+ charged” AA sequence to bind tubulin
- Projection
domain:
- Projects from MT
- Binds to IF, MT or PM.
- Controls spacing of MT
- Found in dendrites or axons
Type II (MAP 2, 4, tau)
- Found in axons or dendrites (depending on the subtype)
- Cross links MT with IF or PM or other MT
- Stabilizes MT during growth, function and division. Tau organizes MT into bundles.
- MAP4 more ubiquitous for Cell division
Some of these MAPs may bind to special sites on the alpha tubulin that form after it is in the microtubule. These are sites where a specific molecule is acetylated or the tyrosine residue is removed from the carboxy terminal. These sites are important marker sites for stabilized microtubules, because they disappear when microtubules are depolymerized.
Motor
Microtubule associated proteins.
This figure shows a 3-D view of a neuron with its processes containing microtubules. At higher magnifications, the vesicles are seen attached to MAPs and moving along the microtubule conveyer belt. The MAPs include kinesins and dynein which "walk" along the microtubules in opposite directions.

The kinesins move the vesicle along towards the plus end and dynein walks towards the minus end. In neurons, as the microtubules grow from the cell body through the processes, the plus end is more peripheral.
These proteins have head regions that bind to
microtubules and also bind ATP. The head domains are thus ATPase motors. The tail domain
binds to the organelle to be moved. ATP is needed for both binding and
movement. Hydrolysis is absolutely essential for movement. It is not known how the energy from ATP breakdown is
converted into vectorial transport.
Kinesin can bind directly to cargo at its light chain end, however dynein requires a complex of proteins. See your text for a list of these proteins and how they bind.
Microtubule motility: Experiments in vitro

One can label beads with kinesin or dyneins
and watch the direction of movement in a cell at the light microscopic level. What would
happen if the beads were simply labeled with "cytoplasmic extract"? This cartoon
shows the motility process in vitro. The tubule is moving along a
negatively charged glass surface and the vesicle moves along the tubule.
Below is an electron micrograph of microtubules linked by MAP bridges (arrows). The star indicates a link between a microtubule and a vesicle. Note the size difference between the vesicle and microtubules.
Drugs that disrupt microtubules.

Colchicine, colcemid, and nocadazol inhibit polymerization by binding to tubulin and preventing its addition to the plus ends. The figure to the right shows this inhibition by colchicine (red). Vinblastine and vincristine aggregate tubulin and lead to microtubule depolymerization. Taxol stabilizes microtubules by binding to a polymer.
For more information, contact:
Gwen Childs, Ph.D.,FAAA
Professor and Chair
Department of Neurobiology and Developmental Sciences
University of Arkansas for Medical Sciences
Little Rock, AR 72205
For questions, contact this email address: