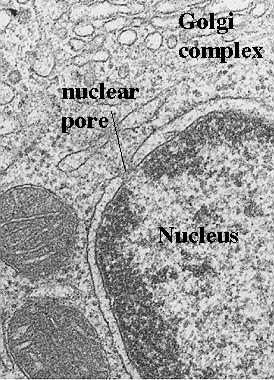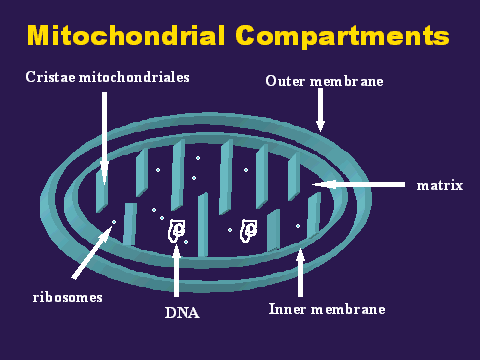
Mitochondria contain two membranes, separated by a space. Both are the typical "unit membrane" (railroad track) in structure. Inside the space enclosed by the inner membrane is the matrix. This appears moderately dense and one may find strands of DNA, ribosomes, or small granules in the matrix. The mitochondria are able to code for part of their proteins with these molecular tools. The above cartoon shows the diagram of the mitochondrial membranes and the enclosed compartments. Return to Menu
The food we eat is oxidized to produce high-energy electrons that are converted to stored energy. This energy is stored in high energy phosphate bonds in a molecule called adenosine triphosphate, or ATP. ATP is converted from adenosine diphosphate by adding the phosphate group with the high-energy bond. Various reactions in the cell can either use energy (whereby the ATP is converted back to ADP, releasing the high energy bond) or produce it (whereby the ATP is produced from ADP).

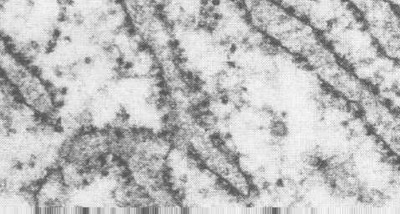
Mitochondria are the cells' power sources. They are distinct organelles with two membranes. Usually they are rod-shaped, however they can be round. The outer membrane limits the organelle. The inner membrane is thrown into folds or shelves that project inward. These are called "cristae mitochondriales". The above electron micrograph taken from Fawcett, A Textbook of Histology, Chapman and Hall, 12th edition, 1994, shows the organization of the two membranes.
For the discussion session, read the following pages in your text: pp 653-676 and 569-672. Alberts et al, Molecular Biology of the Cell, Garland Publishing, Third Edition, 1994
Steps from glycolysis to the electron transport chain. Why are mitochondria important?
Lets break down each of the steps so you can see how food turns into ATP energy packets and water. The food we eat must first be converted to basic chemicals that the cell can use. Some of the best energy supplying foods contain sugars or carbohydrates ...bread, for example. Using this as an example, the sugars are broken down by enzymes that split them into the simplest form of sugar which is called glucose. Then, glucose enters the cell by special molecules in the membrane called “glucose transporters”.
Once inside the cell, glucose is broken down to make ATP in two pathways. The first pathway requires no oxygen and is called anaerobic metabolism. This pathway is called glycolysis and it occurs in the cytoplasm outside the mitochondria. During glycolysis, glucose is broken down into pyruvate. Other foods like fats can also be broken down for use as fuel (see following cartoon). Each reaction is designed to produce some hydrogen ions (electrons) that can be used to make energy packets (ATP). However, only 4 ATP molecules can be made by one molecule of glucose run through this pathway. That is why mitochondria and oxygen are so important. We need to continue the breakdown process with the Kreb’s cycle inside the mitochondria in order to get enough ATP to run all the cell functions.

The events that occur inside and outside mitochondria are diagrammed in
the above cartoon. Pyruvate is carried into the mitochondria and there it is
converted into Acetyl Co-A which enters the Kreb's cycle. This first
reaction produces carbon dioxide because it involves the removal of one
carbon from the pyruvate.
How does the Krebs cycle work?
The whole idea behind respiration in the mitochondria is to use the Krebs cycle (also called the citric acid cycle) to get as many electrons out of the food we eat as possible. These electrons (in the form of hydrogen ions) are then used to drive pumps that produce ATP. The energy carried by ATP is then used for all kinds of cellular functions like movement, transport, entry and exit of products, division, etc. The following explanation is very simple and focuses on only the pathway from pyruvate through the cycle. However, it illustrates the process and its functions.
To run the Krebs cycle, you need several important molecules in addition to all the enzymes. Consult your text for details about the enzymes themselves. This presentation will focus on the electron donors, carriers and acceptors. First, you need pyruvate, which is made by glycolysis from glucose. Next, you need some carrier molecules for the electrons. There are two types of these: one is called nicotinamide adenine dinucleotide (NAD+) and the other is called flavin adenine dinucleotide (FAD+). The third molecule, of course, is oxygen.
Pyruvate is a 3 carbon molecule. After it enters the mitochondria, it is broken down to a 2 carbon molecule by a special enzyme (see text for more details about the biochemistry of each step). This releases carbon dioxide. The 2 carbon molecule is called Acetyl CoA and it enters the Kreb’s cycle by joining to a 4 carbon molecule called oxaloacetate. Once the two molecules are joined, they make a 6 carbon molecule called citric acid (2 carbons + 4 carbons = 6 carbons). That is where the Citric acid cycle got its name....from that first reaction that makes citric acid. Citric acid is then broken down and modified in a stepwise fashion (see text for details) and, as this happens, hydrogen ions and carbon molecules are released. The carbon molecules are used to make more carbon dioxide and the hydrogen ions are picked up by NAD and FAD (see below). Eventually, the process produces the 4 carbon oxaloacetate again. The reason the process is called a cycle, is because it ends up always where it started....with oxaloacetate available to combine with more acetyl coA.
What is “oxidative phosphorylation”?
First, some basic definitions. When you take hydrogen ions or electrons away from a molecule, you “oxidize” that molecule. When you give hydrogen ions or electrons to a molecule, you “reduce” that molecule. When you give phosphate molecules to a molecule, you “phosphorylate” that molecule. So, oxidative phosphorylation (very simply) means the process that couples the removal of hydrogen ions from one molecule and giving phosphate molecules to another molecule. How does this apply to mitochondria?
As the Krebs cycle runs, hydrogen ions (or electrons) are donated to the two carrier molecules in 4 of the steps. They are picked up by either NAD or FAD and these carrier molecules become NADH and FADH (because they now are carrying a hydrogen ion). The following cartoon shows what happens next.

These electrons are carried chemically to the respiratory or electron transport chain found in the mitochondrial cristae (see cartoons above and below this paragraph). The NADH and FADH essentially serve as a ferry in the lateral plane of the membrane diffusing from one complex to the next. At each site is a hydrogen (or proton) pump which transfers hydrogen from one side of the membrane to the other. This creates a gradient across the inner membrane with a higher concentration of Hydrogen ions in the intercristae space (this is the space between the inner and outer membranes).
The following cartoon shows the individual complexes in the electron transport chain. The electrons are carried from complex to complex by ubiquinone and cycochrome C.
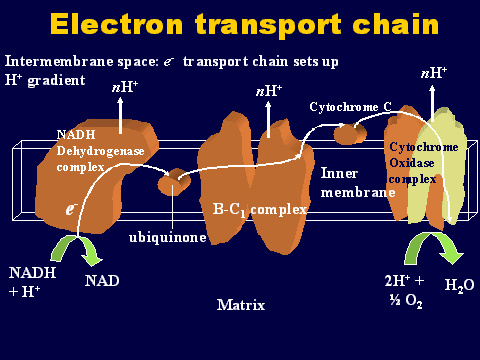
The third pump in the series catalyzes the transfer of the electrons to oxygen to make water. This chemiosmotic pumping creates an electrochemical proton gradient across the membrane which is used to drive the "energy producing machine"...the ATP synthase. This molecule is found in small elementary particles that project from the cristae. The cartoon below shows an elementary particle. Also see its projection from the inner membrane in the previous figure showing the overview of the cristae.

As stated above, this process requires oxygen which is why it is called "aerobic
metabolism". The ATP synthase uses the energy of the hydrogen ion
(also called proton) gradient to form ATP from ADP
and Phosphate. It also produces water from the hydrogen and the oxygen. Thus, each
compartment in the mitochondrion is specialized for one phase of these reactions.
This is how oxidation is coupled to phosphorylation:
To review: NAD and FAD remove the electrons that are donated during some of the steps of the Kreb's or Citric acid cycle. Then, they carry the electrons to the electron transport pumps and donate them to the pumps. So, NAD and FAD are “oxidized” because they lose the hydrogen ions to the pumps. The pumps then transport the hydrogens ions to the space between the two membranes where they accumulate in a high enough concentration to fuel the ATP pumps. With sufficient fuel, they “phosphorylate” the ADP. That is how “oxidation” is coupled to “phosphorylation”.
The hydrogens that get pumped back into the matrix by the ATP pump then combine with the oxygen to make water. And that is very important because, without oxygen, they will accumulate and the concentration gradient needed to run the ATP pumps will not allow the pumps to work.
So, why do we need mitochondria?
The whole idea behind this process is to get as much ATP out of glucose (or other food products) as possible. If we have no oxygen, we get only 4 molecules of ATP energy packets for each glucose molecule (in glycolysis). However, if we have oxygen, then we get to run the Kreb’s cycle to produce many more hydrogen ions that can run those ATP pumps. From the Kreb’s cycle we get 24-28 ATP molecules out of one molecule of glucose converted to pyruvate (plus the 4 molecules we got out of glycolysis). So, you can see how much more energy we can get out of a molecule of glucose if our mitochondria are working and if we have oxygen.
Return to Menu
You can now appreciate the importance of the cristae....not only do they contain and organize the electron transport chain and the ATP pumps, they also serve to separate the matrix from the space that will contain the hydrogen ions, allowing the gradient needed to drive the pump. When the discussion focuses on how mitochondria move proteins into the matrix, you will see another reason why this hydrogen ion (proton) gradient is so important!
As shown in the above cartoons, the molecules in the electron transport
chain are found as a cluster organized
in the cristae. These membrane shelves may be more numerous in mitochondria that are more
active in the production of ATP. Thus, they may increase the density of these membranes as
the need arises. The flight muscle of a hummingbird has many cristae in each
mitochondrion, because the need is so great.
Return to Menu

Mitochondria can be separated and the inner and outer membrane can be
dissociated. This will result in a fraction containing only the inner membrane and the
matrix. These have been called "mitoplasts". They are functional and have helped
us learn more about the compartmentation of mitochondria. One can open mitoplasts and view
the inside membrane surface after negatively staining the membranes. This deposits stain
around any surface projections. With this method, one can see the elementary particles
projecting from the inner surface of the cristae. These are the ATP synthase molecules
(or elementary particles) discussed in the previous section.
The cartoons in the previous section showed cytochrome C lying just outside the inner membrane. It is a loosely attached peripheral protein lying in the space contained by the cristae. In fact, if the outer membrane is removed, often the cytochrome C is lost and must be replaced to promote function of the mitoplast.

How do cytochemists know that cytochrome C is on the inner membrane? We can do cytochemical tests for this cytochrome and the results are shown in this figure. Note that the enzyme reaction product is confined to the cristae and in fact delineates the cristae. Unfortunately, as is the case with most enzyme cytochemistry, the reaction product spreads and it looks like it fills the inter-membrane space. This reflects the orientation of cytochrome C. It is found in space inbetween cristae membranes which suggests it is next to the outer leaflet of the cristae membrane, rather than the inner leaflet (opposite to that of the elementary particles, or ATP synthetase).
For more information, contact:
Gwen Childs, Ph.D.,FAAA
Professor and Chair
Department of Neurobiology and Developmental Sciences
University of Arkansas for Medical Sciences
Little Rock, AR 72205
For questions, contact this email address: